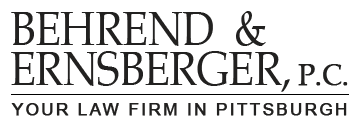Adverse Drug Reactions
Behrend & Ernsberger, PC - Attorneys at Law, Pittsburgh, PA
Have You Experienced Serious Adverse Reactions to a Drug That Were Never Disclosed by the Manufacturer?
Serious or even deadly drug reactions are not known until the first few million consumers try it out, the manufacturer gathers consumer complaints, and the manufacturer amends its product literature to disclose the risk of the infrequent but serious or deadly reactions.
All prescription drugs produce some form of adverse drug reaction, (ADR). Most ADRs are mild, such as a mild stomach upset, and go away on their own. Some ADRs are serious, such as the blistering of the retina of the eye, and persist for months after withdrawal of the medication. A few ADRs cause death.
More on Adverse Drug Reactions
All prescription drugs are tested for ADRs before they are put on the market; but the testing is limited.
Most new drugs are approved with an average of 1500 patient exposures and usually for only relative short periods of time. However, some drugs cause serious ADRs at very low frequencies and would require many more exposures to detect the reaction. To reliably detect the toxic effects of a drug with a 1 in 20,000 adverse drug reaction frequency, a new drug application database would have to include 100,000 patient exposures.
This limited pre-market testing means that the manufacturer probably does not know about the new product’s serious or even deadly drug reactions when the product is put on the market. These serious or even deadly drug reactions are not known until the first few million consumers try it out, the manufacturer gathers consumer complaints, and the manufacturer amends its product literature to disclose the risk of the infrequent but serious or deadly reactions. If this has happened to you, call us at (412) 391- 2515.
More from the FDA on Finding and Learning about Side Effects (adverse reactions).
Other Practice Areas
Select Any One To Learn More
Address: 355 Fifth Ave 12th floor, Pittsburgh, PA 15222
Phone: (412) 391-2503
email: BehrendLawyers@aol.com
Hours: 8 AM – 4 PM (Mon – Fri)
Law Firm Established in 1966
For nearly 55 years, Behrend & Ernsberger’s Lawyers have handled hundreds of important cases for our valued clients.
If you have been injured due to the negligence of others, you have the right to seek the compensation you need and deserve.
Call Behrend & Ernsberger for a free, no obligation consultation regarding your case.